1. INTRODUCTION
Following up the Paris Agreement that ‘all Parties strive to formulate and communicate Long-term low greenhouse gas Emission Development Strategies (LEDS) by 2020’, Korean government declared Korea’s 2050 vision ‘to serve as a stepping stone to reach carbon neutrality by 2050’ [1]. While various scenarios were suggested such as expanding the use of renewable energy sources, direct carbon removal in the industry sector could make a short-term difference by the commercial deployment of future technologies. Be more specific, CO2 emissions from the industry sector account for 55.7% of Korea’s total emissions in 2019, and the steel industry only takes responsible for 31% of the lot (equivalent to 17% of the total) [2]. Therefore, the leading steel company in Korea, POSCO declared its strategy of developing its original HyREX (Hydrogen Reduction Ironmaking) technology to Carbon-neutrality by 2050 [3, 4]. The strategy has to compass the eventual replacement of all blast furnaces to electrical ones to reject any usage of carbon-containing sources, as Hyundai Steel pronounced to establish the HyCube (Hy3; Hyundai Hydrogen Hybrid) production [5]. Nonetheless, a transient technology such as DRI (direct reduced iron) process using COG (coke oven gas) must be imperative in the meantime.
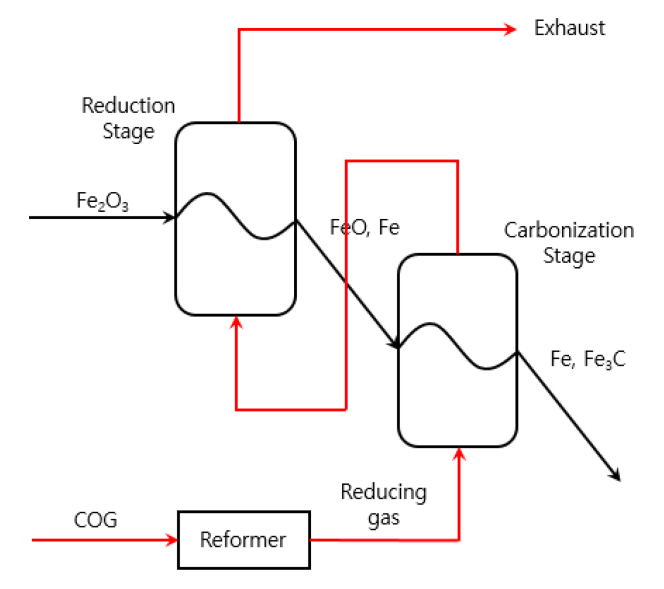
COG, whose typical volumetric composition is 55% H2, 7% CO, 25% CH4 and small amounts of C2H4, CO2, H2O, etc., is a valuable by-product of coal, produced while making the carbon-intensive coke in steel industry [6]. However, only 20% of COG is utilized as fuel and the rest is discharged into the atmosphere (in China, for example), bringing about great environmental consequences as well as substantial energy waste [7-10]. Thus, the companies such as MIDREX Technologies and Praxair have developed processes like Thermal Reactor SystemTM (TRS) using COG to produce DRI products [7]. We also patented a method for manufacturing DRI with multi-stage fluidized bed reduction using reformed COG, as proposed in Figure 1 [11]. The system is composed of two stages (reduction and carbonization), where COG and iron ore (Fe2O3) flow into the system in an opposite (or countercurrent) direction [12, 13]. The reuse of the gas is designed to improve the economics of the system. Iron ore is reduced into iron (Fe) in the 1st reduction stage at ~600 °C and consecutively carbonized into iron carbide (Fe3C, cementite) in the 2nd carbonization stage at ~800 °C [14, 15]. The presence of iron carbide at a level of 2.5~3% is essential in energy-saving for the following smelting step at an electric arc furnace owing to the melting temperature decrease (1538 °C of Fe and 1227 °C of Fe3C). Meanwhile, a high hydrogen content in COG causes low inertia in fluidizing iron ore, so that fine particles of iron ore are required. And a COG reformer controls the hydrogen content in the reducing gas stream. For comparison, the well-known FINEX process has 3 stages plus a pre-stage for drying and preheating the iron ore because the reducing gas from the smelting reduction furnace contains low content of hydrogen and high oxidation degree to decelerate the reduction of iron ore, while 2 stages would be acceptable for the proposed process in Figure 1 with the gas of high reducing capacity. The pre-stage for drying and preheating the iron ore should also be incorporated anyhow.
In this study, we have determined the process parameters of the system proposed in Figure 1, the respective reaction temperature and operating time of two stages, considering the GCR (gas consumption rate) and the constitution of the product at the exit of each stage. The countercurrent flow design with the reuse of the gas reflected the GCR already in that way. The XRD (X-ray diffraction) and EDS (energy dispersive spectroscopy) were employed t°Confirm the constitutional change of iron ore [16, 17]. And because our lab-scale experiments were carried out with a horizontal electric tubular furnace, we have introduced the concept of GOD (gas oxidation degree), the CO2 volumetric content in the reducing gas, to analyze the two-stage process with two separate experiments. In other words, the presence of H2O formed by the reduction of iron ore in an inlet stream is technically implemented by adding equivalent CO2 from a gas bombe in separate experiments.
2. EXPERIMENTAL DETAILS
Carajás iron ore from Para, Brazil was used in this study. The iron ore size was in the range of 0.063~0.125 mm. The chemical composition of Carajas iron ore is shown in Table 1. The iron ore fines were isothermally reduced by a mixed gas using a horizontal muffle box open type tube furnace (AJ-MBOT3, 80 mm in diameter, 600 mm in length, Ajeon Heating Industrial Co. Ltd., Korea). A quartz tube was used as a reactor. The temperature of the furnace was controlled by a PID controller with an accuracy of ±1 °C. For the reduction of iron ore, a mixed gas of H2, CH4, and CO2 with a total flow rate of 5 L/min was used for all runs. An alumina boat loaded with 6 g of iron ore sample was placed in the middle of the tube and Ar gas was introduced at a flow rate of 2 L/min when heating the furnace to the wanted reaction temperature and cooling it to room temperature. The mass change of samples after the reaction was measured by an electronic balance with the sensitivity of 0.01 g. Then, the phase of the sample was analyzed by an X-ray diffractometer (Ultima IV, RIGAKU, Japan) and its atomic composition was obtained by an energy dispersive spectroscopy (EDS) detector implemented in a field-emission scanning electron microscope (JEM-2100F, JEOL, Japan).
Meanwhile, thermodynamic calculations were also carried out using the CALPHAD (CALculation of PHAseDiagrams) method with a commercial software known as ‘Therm°Calc’ [18]. In this method, the elements are defined for a given system and the thermodynamic properties of all available species involving defined elements are collected from a database as forms of Gibbs energies. The parameters of the mathematical models were then evaluated by optimizing the model fit to the collected information. The SSUB5 database was used, which is one of the largest SGTE (Scientific Group Thermodata Europe) databases, containing 5580 pure condensed compounds and gaseous species.
3. RESULTS AND DISCUSSION
3.1 Determination of reaction temperatures at reduction and carbonization stages
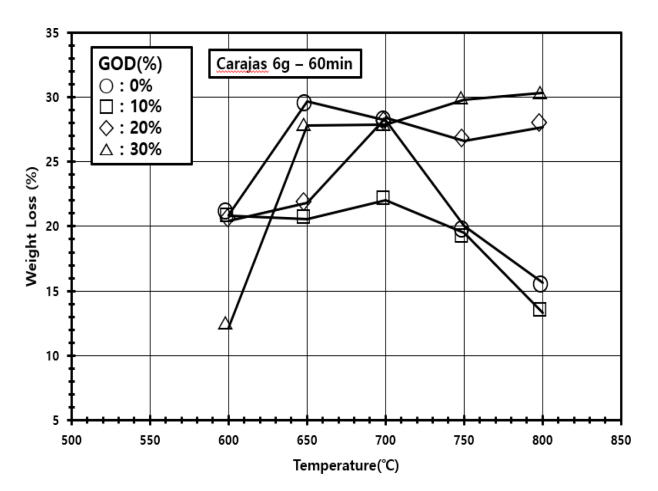
The weight loss of the sample was monitored as the temperature increased, with respect to various GOD (gas oxidation degree) values, as shown in Figure 2. GOD is defined as the volumetric content of CO2 in reducing gas to represent the oxidation state of the gas.
The GOD value of the reducing gas depends on the operation of COG reformer in a real process (see Figure 1), but the inlet compositions of gas mixtures containing H2, CO2, and CH4 with no trace of H2O were controlled by adjusting gas flow rates in our experiments. One-stage horizontal furnace experiments with the above inlet composition control are however limited in analyzing the proposed two-stage process (see Figure 1) directly, because the reducing capacity decrease of the exhaust gas from a stage is resulted from the formation of H2O by the reduction of iron ore. Therefore, we come up with an idea of replacing H2O by CO2 artificially, so that the decreased reducing capacity of the gas from the carbonization stage is accounted for. Because the resulting stable H2O will not participate in further reactions, its replacement by the similarly stable CO2 is justified. At this point, how much H2O is formed in the stage was not exactly analyzed, but instead we adopted an empirical suggestion that the oxidation degree in the gas stream at each stage is increased by about 10% in the FINEX process (will be discussed in the section 3.2).
As shown in Figure 2, the temperature range was from 600 to 800 °C and the reaction time was 60 min with 6 g of Carajás iron ore (Fe2O3). The GOD was controlled from 0 to 30% by adjusting the volumetric contents of H2 and CO2 with the content of CH 4 fixed at 30%. The weight loss combined with the reaction from Fe2O3 to FeO or Fe was 20% even with low GOD values at 600 °C, presenting that the reduction of iron ore is not sufficiently fulfilled in terms of kinetics. However, it increased up to 28~30% at 600~700 °C with low GOD values. Considering that the maximum attainable weight loss from Fe2O3 to Fe by stoichiometry is 30%, the reduction of iron ore is successfully completed at those conditions. Meanwhile, as the temperature was increasing through 750 to 800 °C, an intriguing pattern of weight loss came out. The weight loss decreased down to 15~20% at low GOD values of 0 and 10% while it retained with high GOD values, suggesting that the carbonization of Fe to Fe3C is possible only at high temperature and low GOD conditions. Therefore, the results give rationale for the design of our proposed countercurrent process shown in Figure 1. The reformed reducing gas having low GOD moves into the carbonization stage operated at a high temperature such as 800 °C, completing the reduction of iron ore exited from the reduction stage and the following carbonization. Then, although the exhaust from the carbonization stage flows into the reduction stage with an increased GOD, it has no effect to the initiation and progress of the reduction of iron ore in the reduction stage operated at a lower temperature such as 600 °C. The countercurrent scheme is also advantageous in energy-saving in itself.
3.2. Determination of operating times at reduction and carbonization stages
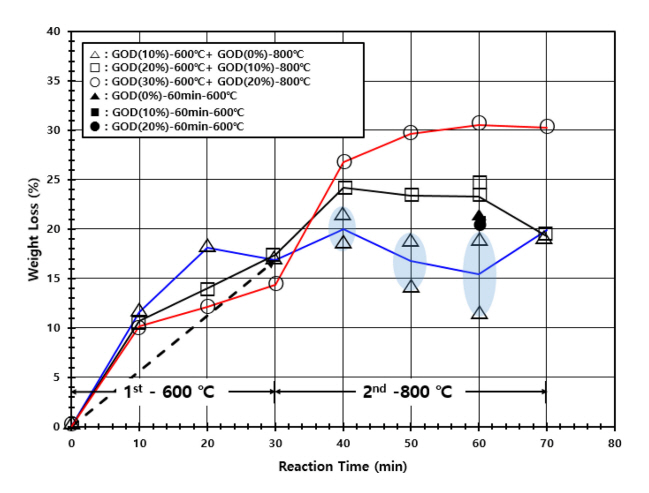
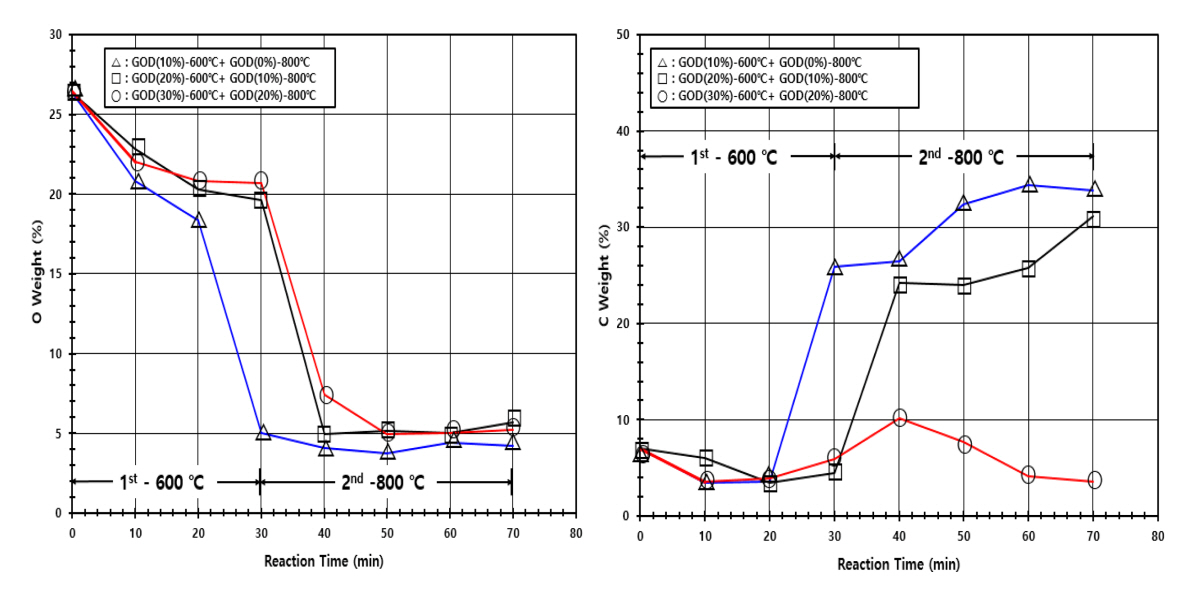
The previous DOE (design of experiments) in Section 3.1 determined the operating temperatures of two stages with a sufficiently long operating time of 60 min; 600 and 800 °C at the reduction and carbonization stages, respectively. Now, the optimal operating time at each stage is determined similarly by monitoring the weight loss of the sample as the time increases, with respect to various GOD values, as shown in Figure 3. The experiments were executed by the following way. A horizontal furnace was maintained at 600 °C for the 1st reduction of iron ore occurred in the reduction stage, where samples were collected for the analysis every 10 min up to 30 min. Then, to find the optimal time for the 2nd reduction and carbonization occurred in the carbonization stage, a horizontal furnace was maintained at 600 °C for 30 min for the 1st reduction of iron ore and the temperature was raised to 800 °C under an inert (N2) atmosphere before samples were collected for the analysis every 10 min up to 40 min during the 2nd reduction and carbonization. The longest operating time was 70 min in total; 30 min at 600 °C and 40 min at 800 °C. Meanwhile, the GOD value was controlled by adjusting the content of CO2 from a gas bombe. When the temperature of the furnace was raised to 800 °C, the GOD value was lowered by 10% to equilibrate it to the oxidation state of the actual gas stream. About 10% GOD increase in the gas stream was detected empirically at each stage in the countercurrent multi-stage FINEX process.
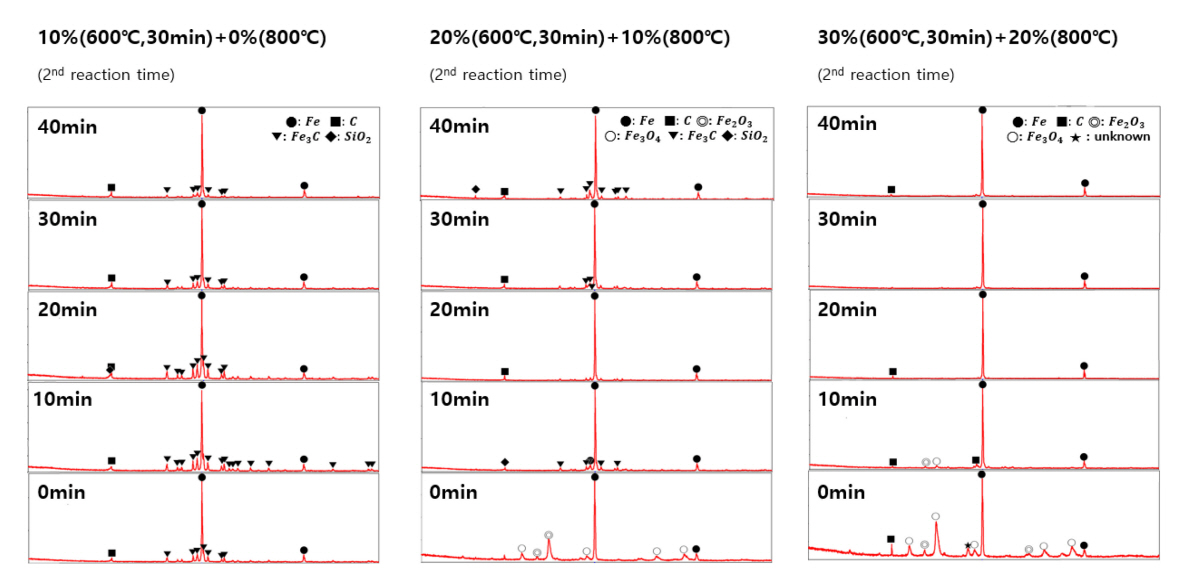
As shown in Figure 3, the weight loss is 15~18% by the reduction for 30 min with that the weight loss is proportional to the reduction time and inversely proportional to the GOD value. XRD (see Figure 4) confirmed the presence of Fe3O4 and Fe2O3 phases with a high GOD value (20 and/or 30%), presenting that the reduction of iron ore is in progress (Fe2O3 → Fe3O4 → FeO → Fe). And Fe3C and C phases were observed instead with a low GOD value (10%), suggesting that the reduction of iron ore proceeds far more and even carbonization occurs partially under a higher reducing environment. XRD spectra at the lowest row in Figure 4 are of the samples reduced only for 30 min at 600 °C for different GOD values. Accordingly, an elemental analysis using EDS (see Figure 5) reported the decrease of oxygen and the increase of carbon as the GOD value increases; 20 wt.% oxygen and 2 wt.% carbon at the GOD of 20~30%, compared to 5 wt.% oxygen and 26 wt.% carbon at the GOD of 10%.
The weight loss showed a distinct trend with respect to GOD values during the 2nd reduction and carbonization. All the samples were treated at 600 °C for 30 min a priori and consecutively cured for up to 40 min with 10% lowered GOD values. The weight loss increased rapidly for the additional 10 min (equivalent to 40 min in Figure 3) and saturated at 30% after 20 min with the GOD value of 20%, while XRD spectra in the 3rd column of Figure 4 confirmed the completion of reduction of iron ore by the presence of Fe only after 20 min. And EDS reported low contents of oxygen and carbon by 5 wt.% and 2 wt.%, respectively. Meanwhile, at a low GOD value (0 and/or 10%), there appeared a maximum of weight loss in 10 min and it decreased to 20% with the time (see Figure 3). The phases of Fe3C and C other than Fe were detected after 40 min (equivalent to 70 min in Figure 3) and the contents of carbon became 30~33 wt.% with a low 4~5 wt.% of oxygen by EDS (see Figure 5).
In summary, the desirable operating times of the reduction and carbonization stages are 30 and 40 min, respectively, and the low GOD of ~10% in reducing gas flown into the carbonization stage (see Figure 1) is required to achieve the wanted reduction and carbonization of iron ore at given operating times.
3.3. Verification of experimental results by using equilibrium analysis
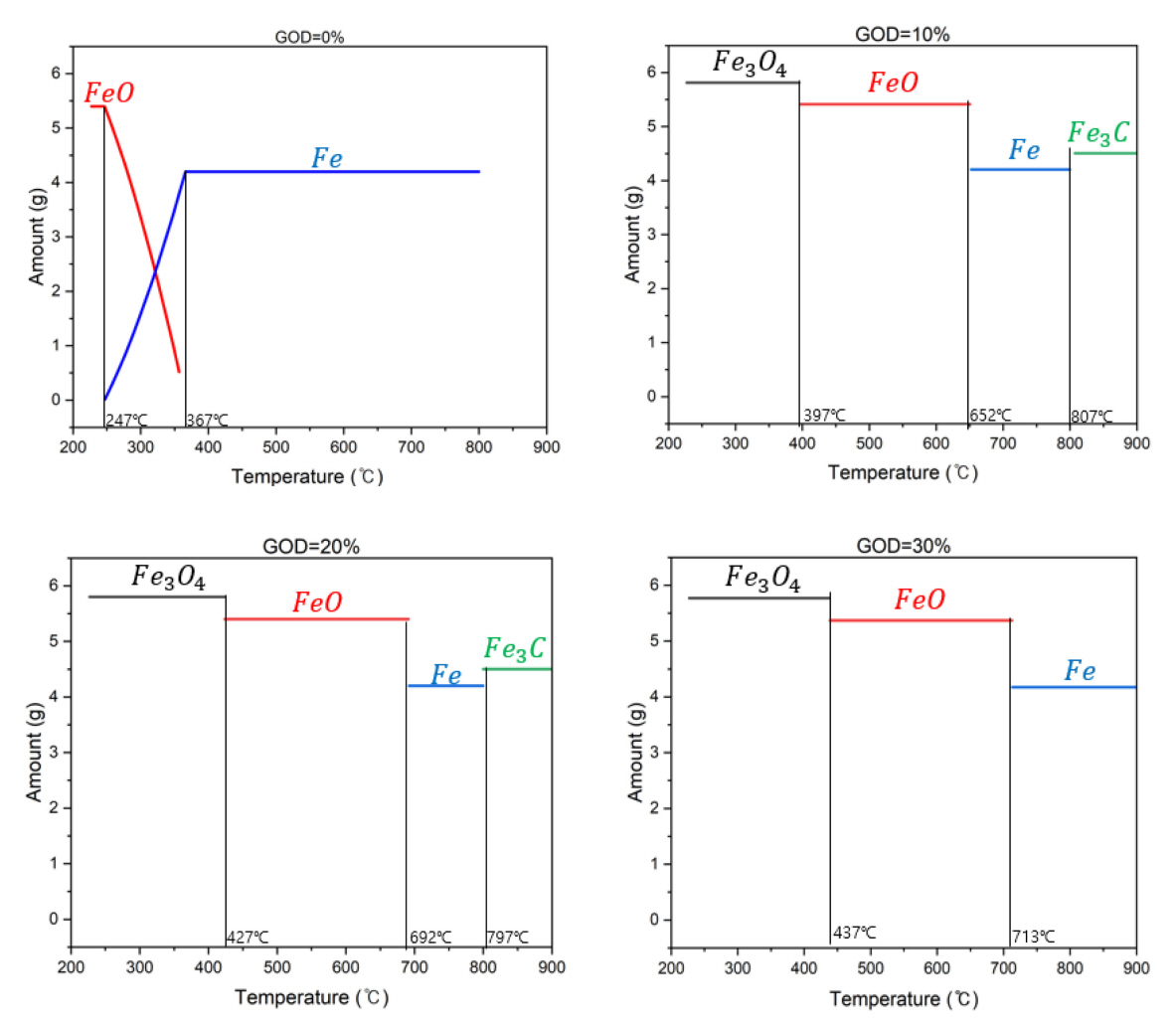
An equilibrium analysis was performed by the ‘Therm°Calc.’ software based on the experimental conditions that Fe2O3 was 6 g and the total gas flow rate was 5 L/min with the fixed content of CH4(g) at 30%. The GOD was changed from 0 to 30%, presenting that H2 (g) was replaced by CO2(g) with respect to the GOD values. For example, the flow rates of H2(g) and CH4(g) were 3.5 and 1.5 L/min at GOD = 0%, respectively, and those of H2(g), CH4(g), and CO2(g) were 3.0, 1.5, and 0.5 L/min at GOD = 10%, respectively. Then, the amount of each gas species for calculations was set by multiplying the flow rate of each species by 60 min based on the reference operating time of 60 min. Calculated results of the constitutional change in solid during the reduction and carbonization of iron ore versus the reaction temperature with respect to various GOD values (0, 10, 20, and 30%) are given in Figure 6.
With the GOD of 0%, the stable phase in solid is FeO even at a low temperature of 247 °C and reduced to Fe at around 367 °C, which is attributed to a high degree of reducing environment created by a high content of H2(g). During this reduction, CH4(g) does not contribute and is just decomposed to C(graphite) and H2(g) at about 527 °C. Then, C(graphite), not FE3C, is incorporated into the constitution of solid phase and the weight loss of the sample increases in a high temperature range as shown in Figure 2. The 1st column in Figure 4 shows the change of crystalline phases of the sample, which is reduced at 600 °C and 10% GOD for 30 min a priori and consecutively carbonized at 800 °C and 0% GOD up to 40 min. The presence of CO2 (10% GOD) even at a low temperature of 600 °C probably contributes to the formation of FE3C phase in the sample (see the bottom of the 1st column in Figure 4). And interestingly the intensity of XRD peaks of FE3C phase decreases as the operating time reaches to 40 min at 0% GOD, suggesting that only H2 environment (GOD 0%) starts to drive the system to equilibrium. Relevant reactions are as follow [19, 20] and the effect of the presence of CO2 for the formation of FE3C phase is discussed next.
With the GOD of 10 and 20%, the stable phase in solid is Fe3O4 at 247 °C and reduced to FeO and consecutively to Fe. The reduced Fe is now carbonized to Fe3C at about 797 °C and relevant reactions are in Reactions (4)-(6). At a high temperature above 527 °C, CH4(g) is cracked to C(graphite) which reacts with CO2(g) to produce CO(g). The reduced Fe is carbonized to Fe3C by CO(g). However, the formation of Fe3C is restrained with the GOD of 30% because a high content of CO2(g) limited the Reaction (6) in a forward direction thermodynamically [21].
The equilibrium analysis also explained our experimental results shown in Figure 4 properly, with that Fe2O3 is reduced to Fe3O4, FeO and Fe consecutively in a temperature range from 600 (reduction stage) to 800 °C (carbonization stage), and Fe3C is formed with a low GOD value during the carbonization stage. Meanwhile, the cracked C(graphite) and CO(g) contributes to the reduction of Fe2O3 additionally by the following reactions.
4. CONCLUSION
This study was aimed to determine the operating parameters of the proposed two-stage DRI process by analyzing the weight loss of the iron ore samples depending on the reaction temperature and operating time. In doing so, a GOD (gas oxidation degree) was introduced to overcome the limit of our one-stage experimental setup and supplementary analysis techniques like XRD and EDS as well as equilibrium analysis were employed. Our study is expected to be a guideline for the optimization of relevant DRI processes in view of economics; for example, the lowest temperature range (< 800 °C) of the 2nd carbonization stage is worthwhile to be searched to improve the overall energy unit price of the process.
1) With the operating time fixed at 60 min, the correlation between the reaction temperature and GOD value was investigated. In general, the reduction of iron ore is completed at ~700 °C irrespective of the GOD value. However, the carbonization of reduced iron (the formation of Fe3C) at a high temperature of 800 °C is possible only with low GOD values. The lower the GOD value becomes, the higher the reducing environment is. Those results justified the countercurrent scheme in the proposed two-stage process. The reduction of iron ore initiates in the 1st stage at a low temperature of ~600 °C and the GOD value is irrelevant in this step. Then the reduction and the subsequent carbonization is completed in the 2nd stage at a high temperature of ~800 °C with a low GOD.
2) With the reaction temperature fixed at 600 °C in the 1st stage and at 800 °C in the 2nd stage, the correlation between the operating time and GOD value was investigated. The optimal operating time for the 1st stage is 60 min and 40 min for the 2nd stage only with a high reducing environment (i.e., reducing gas having a GOD less than 10%).
3) An equilibrium analysis was performed with actual experimental conditions applied. The reducing gas with too low GOD (in other words, too high reducing environment) cannot generate CO(g), the essential reactant for the Fe3C formation. The CO(g) is the product of the reaction between the cracked C(graphite) from CH4(g), and CO2(g). Then it forms Fe3C by the reaction, ‘3Fe + 2CO (g) ↔ Fe3C + CO2(g).’ Thus, the reducing gas involving too much CO2(g) inhibits the formation of Fe3C by Le Chatelier’s principle.